Why is silicon one of the most useful elements?
A piece of high school Chemistry homework led to an in-depth exploration of the element silicon last month. Tasked with creating a leaflet about an element, Silicon was perhaps not the obvious choice but it was a learning experience for both the student, and the parent.
Silicon was discovered by Jöns Jacob Berzelius in 1824 and has even had an entire southern region of San Francisco named after it, Silicon Valley. The name was first adopted in the early 1970s because of the region’s association with the silicon transistor, which is used in all modern microprocessors.
Silicon or Silicone?
Silicon is a metalloid, with the properties of both metals and non-metals. Its greatest quality is probably its ability to conduct electricity, especially at high temperatures. What silicon isn’t though, is silicone. Silicone is a polymer made of elements, usually silicon, carbon, hydrogen and oxygen. Instead of being a semiconductor, silicone is an electric insulator. Source: werecyclesolar.com
The Royal Society of Chemistry describes Silicon as ‘one of the most useful elements to mankind’. It makes up 27.7% of the Earth’s crust by mass, the second most abundant element in the earth’s crust and the seventh most abundant element in the universe, but why are we talking about it on the Like Technologies website?
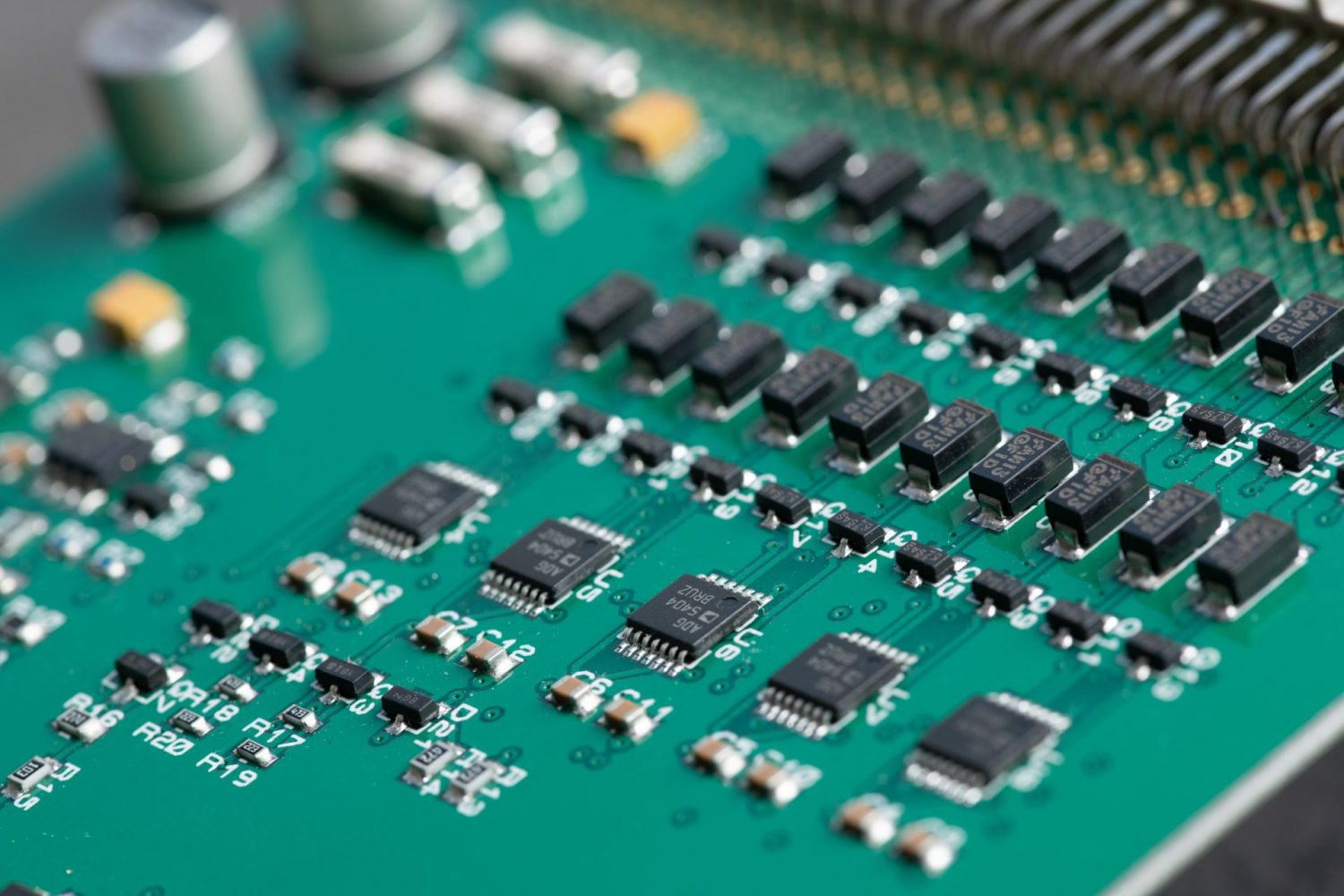
Silicon is a material commonly used as a semiconductor in electronics. Transistors, printed circuit boards, and integrated circuits make use of silicon’s highly conductive properties to maximise their performance. It’s in computer chips, liquid crystal displays, diodes and much more. Did you know that silicon is also the major component of solar cells and is vital to the renewable energy industry?
Even though it’s the second most abundant element in the earth’s crust, oxygen being the first, it does not occur as a pure state in nature. It occurs as a compound as an oxide (silica SiO2) or silicates (SiO4). The oxide includes sand, quartz, rock crystal, amethyst, agate, flint and opal. The silicate form includes asbestos, granite, hornblende, feldspar, clay and mica.
Berzelius made the discovery in the 18th century by heating chips of potassium in a silica container and then carefully washing away the residual by-products. Today, high-purity silicon, for the electronics industry, is prepared by the thermal decomposition of ultra-pure trichlorosilane, followed by recrystallisation.
Our workshop is currently a hive of activity as we refurbish hundreds of PCBs all containing this vital element and it has attracted some recent attention due to concerns around the disposal of solar panels. Silicon can be recycled but at a cost. According to Recycle PV Solar’s Sam Vanderhoof, only 10% of solar panels are being recycled, with 90% going to landfills. As reported by Green Journal;
“Solar panels are usually made of materials that are either recyclable or reusable. Components such as glass and specific metals make up about 80% of the solar panels’ mass, and are relatively easy to recover. Similarly, polymers and electronic components in solar panels can also be recycled. But the reality of solar panel recycling is more complicated than just taking them apart and reusing their components. The recycling processes at work right now are not very efficient. This means recovering the materials can cost more than manufacturing a new panel.”
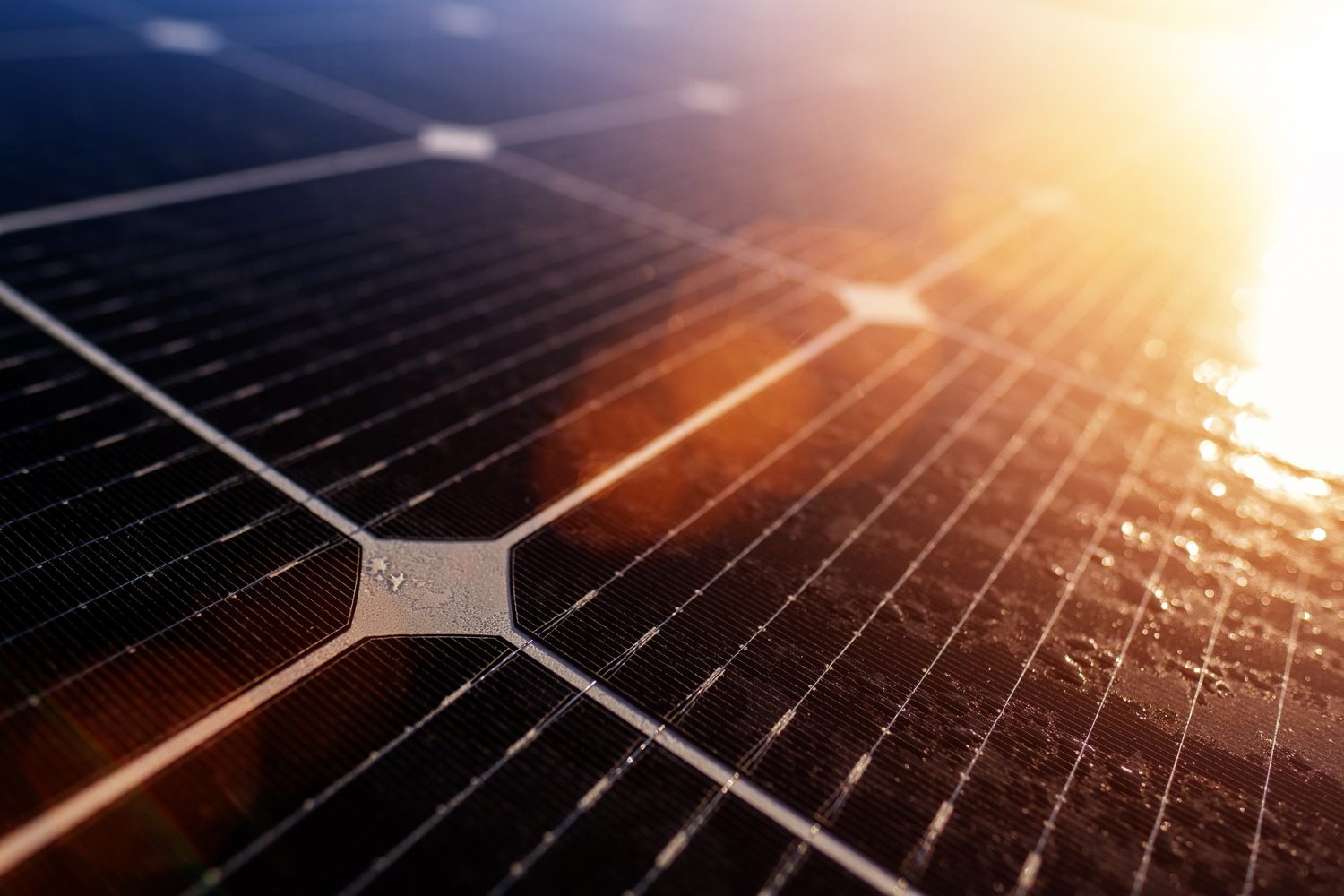
“Economic incentives are rapidly aligning to encourage customers to trade their existing panels for newer, cheaper, more efficient models. In an industry where circularity solutions such as recycling remain woefully inadequate, the sheer volume of discarded panels will soon pose a risk of existentially damaging proportions….Of all sectors, sustainable technology can least afford to be shortsighted about the waste it creates. A strategy for entering the circular economy is absolutely essential — and the sooner, the better.” Harvard Business Review.
A quick internet search throws up an article which explores the process of recycling silicon wafers which can be viewed by clicking here and there is research happening around environmentally friendly silicon recycling which can be viewed here – https://www.labnews.co.uk/article/2030843/environmentally-friendly-silicon-recycling
How much of this useful element is currently lying dormant in landfill?

